 A new study into the cleanliness of endoscopes following high level disinfection has revealed some important findings. The research team, headed by Dr. Adarsh Thaker at UCLA, used specialized digital video cameras to perform visual inspection on 59 duodenoscopes, gastroscopes, echoendoscopes and colonoscopes after manual cleaning and high level disinfection.
A new study into the cleanliness of endoscopes following high level disinfection has revealed some important findings. The research team, headed by Dr. Adarsh Thaker at UCLA, used specialized digital video cameras to perform visual inspection on 59 duodenoscopes, gastroscopes, echoendoscopes and colonoscopes after manual cleaning and high level disinfection.
Each visual inspection included a 90- to 120-second video recording performed by endoscopy technicians. This article by Caroline Helwick at Gastroenterology & Endoscopy News summarizes the findings from the research:
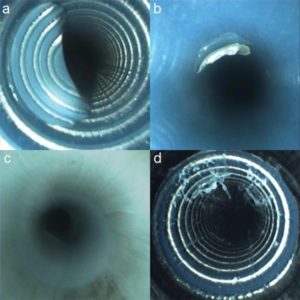
“Five expert endoscopists reviewed 97 videos and scored each for moisture, debris, stains or discoloration, scratches and channel shredding—damage that results in strips or filaments of the channel protruding into the lumen. All scopes were less than 3 years old, and during the study period 16 new devices were delivered to the unit.
Even the new instruments were not immune to damage. “The most common finding in all our devices was scratches, followed in equal amounts by shredding and discoloration,” Dr. Thaker reported (Figure). Debris and moisture were also seen but to a lesser degree.
All types of scopes revealed scratches (86%), shredding (59%) and discoloration (59%). Debris was observed less often (24%) and was seen in none of the colonoscopes. Although moisture and fluid were observed infrequently overall (8% of inspections), it was not uncommon in gastroscopes (36%) and colonoscopes (33%) and was not visible in duodenoscopes or echoendoscopes. Nor was simethicone evident.
“Strikingly, the scopes that underwent forced air dry had zero evidence of visible moisture the next day, compared to five of 18 scopes that underwent overnight passive ventilation alone,” he said.
Among the new devices, two of four new duodenoscopes and nine of 12 new echoendoscopes had visible scratches, and one of four new duodenoscopes and eight of 12 new echoendoscopes had shredding, according to Dr. Thaker. “None were found within two weeks of delivery, but between two and four weeks we had positive findings on inspection,” he said.
No clinical infections were identified in his center during the study period. Whether the imperfections observed in this study present a risk for pathogens or infection remains unknown, he added.
“One thing we think is clear from our study is that forced air dry—a simple two-minute process—is effective in eliminating moisture, which has been implicated as a factor in contamination. Maybe we should incorporate this more broadly,” he said.”
Read the entire article here: Expect the Unexpected Inside Those Scopes
While endoscopes continue to be a focal point of safety issues for hospitals nationwide, both the manufacturers and the FDA believe that the scopes currently in market are usable as long as hospitals follow the manufacturer reprocessing instructions exactly. Hospitals that are concerned about staff adherence to instructions for use can turn to automation systems such as iRIScope to get better control of high-risk processes such as endoscope reprocessing. These systems improve staff compliance by standardizing processes and automating many of the data collection workflows. As endoscopes continue to be evaluated for their safety and risks of infection, hospitals must be diligent in their ability to consistently follow manufacturer cleaning instructions every single time to avoid potential litigation.
